Introduction
Lithium tantalate (LiTaO3, referred to as LT), as an excellent multifunctional crystal material, has good piezoelectric, electro-optical and pyroelectric properties, and is ideal for making surface acoustic wave (SAW) filters, resonators, tuners, Q switches and pyroelectric detectors. Devices made from LT crystal (www.wisoptic.com) are widely used in the automotive electronics, 5G communications and infrared detectors, and have broad market prospects.
In 1965, Ballman used the pulling method to grow LT single crystal for the first time. As a ferroelectric crystal, LT is colorless or light yellow, belongs to trigonal crystal system, with density 7.45 g/cm3, Curie temperature 603 °C, melting point 1650 ° C. It’s TCD (Temperature Coefficients of Delay ) is 18×10-6 in temperature range 20-80 ° C. LT crystal is rich in raw material, stable in performance, easy to process, and capable of preparing high-quality, large-size single crystals. The current market demand of LT crystal is keeping increasing. This paper introduces the characteristics, performance regulation methods and crystal preparation methods of LT crystal, analyzes its research progress in various application fields, and looks forward to its future.
1.1 Crystal Structure and Defects
LT is trigonal crystal, and each hexagonal unit cell contains 6 LiTaO3 molecules. Table 1 shows the atomic coordinates of the hexagonal unit cell of LT crystal. LT crystal has ilmenite structure and an ABO3 lattice with an oxygen octahedral framework. Miyazawa et al. found that the solid-liquid isotropic point of LT is at the Li2O mole fraction of 48.75%, that is, when the molar ratio r (Li)/r (Ta) = 48.75/51.25 = 0.951. The crystal lattice constant of LT crystals is a=b=0.5154nm, c=1.3863nm.
Table 1. Atomic coordinates of the hexagonal unit cell of LT crystal
|
atom |
x / nm |
y / nm |
z / nm |
|
Ta |
0 |
0 |
0 |
|
Li |
0 |
0 |
0.2821 |
|
O |
0.0534 |
0.03396 |
0.0695 |
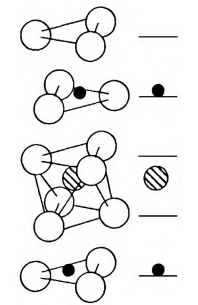
In the LT crystal, the amount of Ta5+ is more than the amount of Li+. Because the bond of Ta-O is tighter than that of Li-O, therefore the excess Ta5+ will occupy the site of Li+, in general. In order to maintain the electrical neutrality of the crystal, vacancies on the site of Li+ will appear. But sometimes Li+ occupies the Ta5+ site. So there are two intrinsic defects in LT crystal: anti-site tantalum (TaLi4+) and lithium vacancies (VLi-). Compared with lithium niobate, lithium tantalate has fewer intrinsic defects. Figure 1 shows the crystal structure model of lithium tantalate.
Figure 1. The crystal structure model of lithium tantalate
Post time: Jul-29-2023